Research topic
The genus Pestivirus
Bovine viral diarrhea virus (BVDV-1, BVDV-2), Classical Swine Fever virus (CSFV) and border disease virus (BDV) of sheep form the genus Pestivirus in the family Flaviviridae.These viruses are economically highly important pathogens of live stock industries. This family also comprises the genus Flavivirus with members like tick borne encephalitis virus, yellow fever virus and dengue virus and the genus Hepacivirus represented by human hepatitis C virus (HCV). Currently members of these three genera are under investigation in scientific projects in the institute.
BVDV is of high economic impact by causing massive losses in stock farming industries. Infection of pregnant animals frequently leads to malformations, abortions or the birth of calves persistently infected by BVDV for their entire lifespan. Therefore, BVDV is a model for persistent viral infection.
Another peculiarity of pestiviruses are recombination events at the RNA level. In those cases fragments of viral mRNAs are integrated into the viral RNA genome. The resulting viruses encode in addition to the viral polyprotein a host cell protein, e.g. ubiquitin. This leads to an altered polyprotein processing scheme causing the deregulation of viral replication. In the context of the persistent infection RNA recombination ist connected to the death of the animal. These events depict that RNA viruses have access on the entire mRNA pool of their host for viral evolution. Based on these facts pestiviruses are models for virus evolution.
Pestiviruses are small (40-60 nm) enveloped viruses, with a single stranded RNA genome of positive polarity. Protein expression occurs via translation of a single large polyprotein which is co- and posttranslationally processed by cellular and virus-encoded proteases into the functional virus proteins. Bovine viral diarrhea virus (BVDV) serves as model virus.
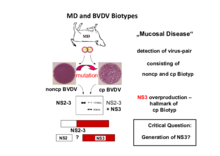
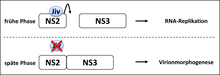
Investigations concerning processing of the viral polyprotein and the enzymes involved are one of the main topics of our group. Processing of nonstructural protein 2-3 (NS2-3) is of special interest, since the extent of NS2-3 cleavage correlates with pestiviral cytopathogenicity. Accordingly, pestivirus strains which show efficient NS2-3 cleavage are cytopathogenic (cp) while the ones which show only inefficient NS2-3 cleavage are noncytopathogenic (noncp). Recent investigations have demonstrated a protease activity in pestiviral NS2; the regulation of this protease and its influence on pestiviral replication and cytopathogenicity are central points of our current work. A goal of ongoing investigations is the identification of additional factors involved in the regulation of pestiviral replication.
In this context our group identified a cellular chaperone of the family of J-domain proteins which stably interacts with pestiviral NS2 and stimulates NS2-3 cleavage. Therefore, we termed this protein "J-domain protein interacting with viral protein" (Jiv). Cells overexpressing Jiv show efficient NS2-3 cleavage and the development of a cytopathogenic effect even upon infection with noncp pestivirus strains. We could demonstrate that Jiv (DNAJ-C14) represents an essential cofactor of the pestiviral NS2 protease. Limiting amounts of Jiv in the natural host cell is a crucial prerequisite for the noncp biotype of the virus and thereby its ability to establish a persistent infection. Tissue specific regulation of Jiv expression and its effect on pestiviral replication are major issues of our current work.
Interestingly, pestiviruses require for virion morphogenesis uncleaved NS2-3 which is a basic difference to the situation in the HCV system. Recently we could demonstrate that pestiviruses can adopt to viron morphogenesis in the absence of uncleaved NS2-3. The molecular basis of pestiviral virion morphogenesis in the presence and absence of uncleaved NS2-3 is a topic of ongoing research.
Current projects also address the roles of different other NS proteins like NS5A in pestiviral replication.